Auxergen Achieves 2024 Qualified Maryland Biotechnology Status
Baltimore, Maryland – March 11, 2024
Auxergen, Inc. has achieved Qualified Maryland Biotechnology Company (QMBC) status for 2024. QMBC status provides early-stage biotechnology companies with refundable tax credits to help offset high research and development costs.
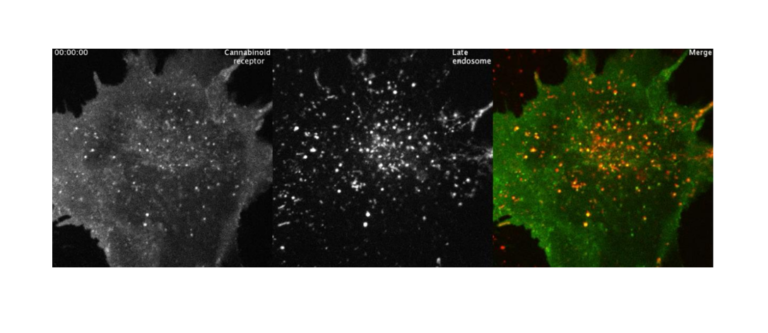
Auxergen develops phage-based cures for agricultural, environmental, and human diseases. The company is currently fielding a bio-control solution to combat the bacterial blight, Xylella fastidiosa, as well as a cannabis biokinetic analysis tool (CBK assay), a bio-antiviral dry fogging technology, and new phage display libraries. Auxergen has also published in diverse fields such as evolutionary virology, epidemiology, plant pathology and molecular cell biology.
“Auxergen’s prolific scientific output, including the development of eight unique technologies in human, plant, and environmental health, is a testament to the QMBC program’s support,” says Gregory Contreras, Auxergen’s C.E.O. “Since 2019, we’ve published a dozen high-impact papers and three patents. The $600k raised through QMBC allows us to advance fundamental science and nurture the next generation of scientists.”
“Auxergen helps bridge the school-to-work gap,” said Dr. Ting-Yu Yeh, co-founder and C.S.O. “Since 2020, our interns have gained admission to prestigious universities like Cornell, Georgetown, SUNY Binghamton, and UC Santa Barbara, while gaining valuable business skills at the C-suite level.”
Learn more about Auxergen’s innovative work at https://auxergen.com and watch short videos on phages and XylePhage.
# # #
Auxergen, Inc. is a dedicated team of scientists and visionaries committed to developing groundbreaking solutions for critical global challenges. The company is proud to join other renowned QMBC recipients like Martek Biosciences, Human Genome Sciences (GSK), and Kite Pharma (Gilead Sciences) – all leaders in the field of human health.
Media Contact:
Michael Feehley
michael@auxergen.com
443.929.9757